Civil Action Complaint Template

A standardized form used to initiate lawsuits in non-criminal cases provides a framework for presenting claims against another party. This framework ensures all necessary information, such as the parties involved, ...
Read more
Complaint Action Plan Template
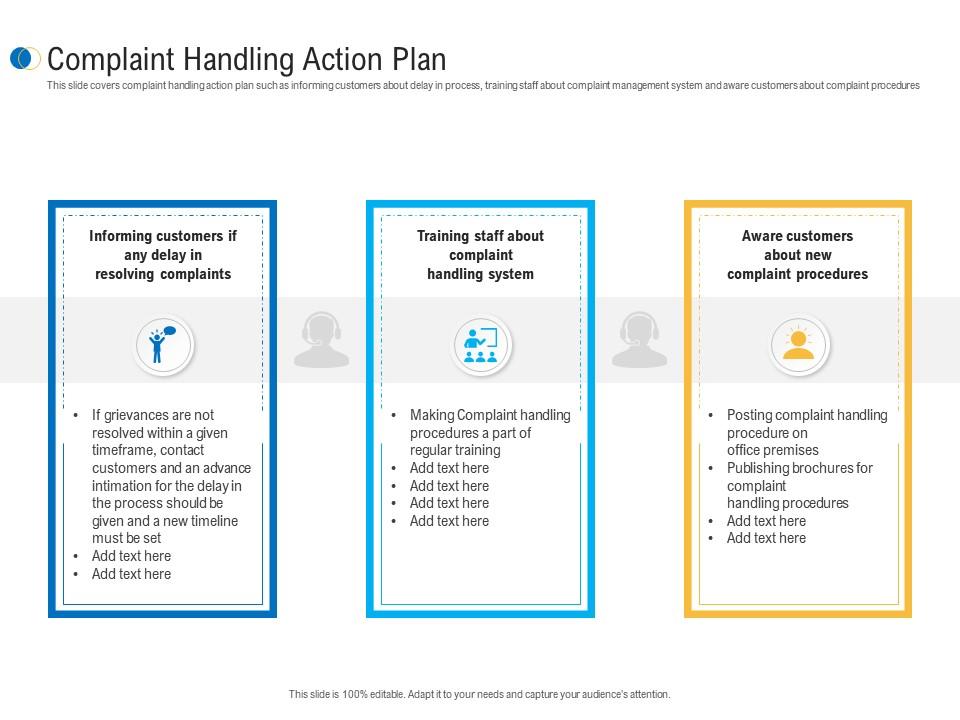
A structured framework for addressing customer dissatisfaction provides a methodical approach to resolving issues and improving service quality. It typically outlines steps such as acknowledging the complaint, investigating the cause, ...
Read more
Corrective and Preventive Action Procedure Template
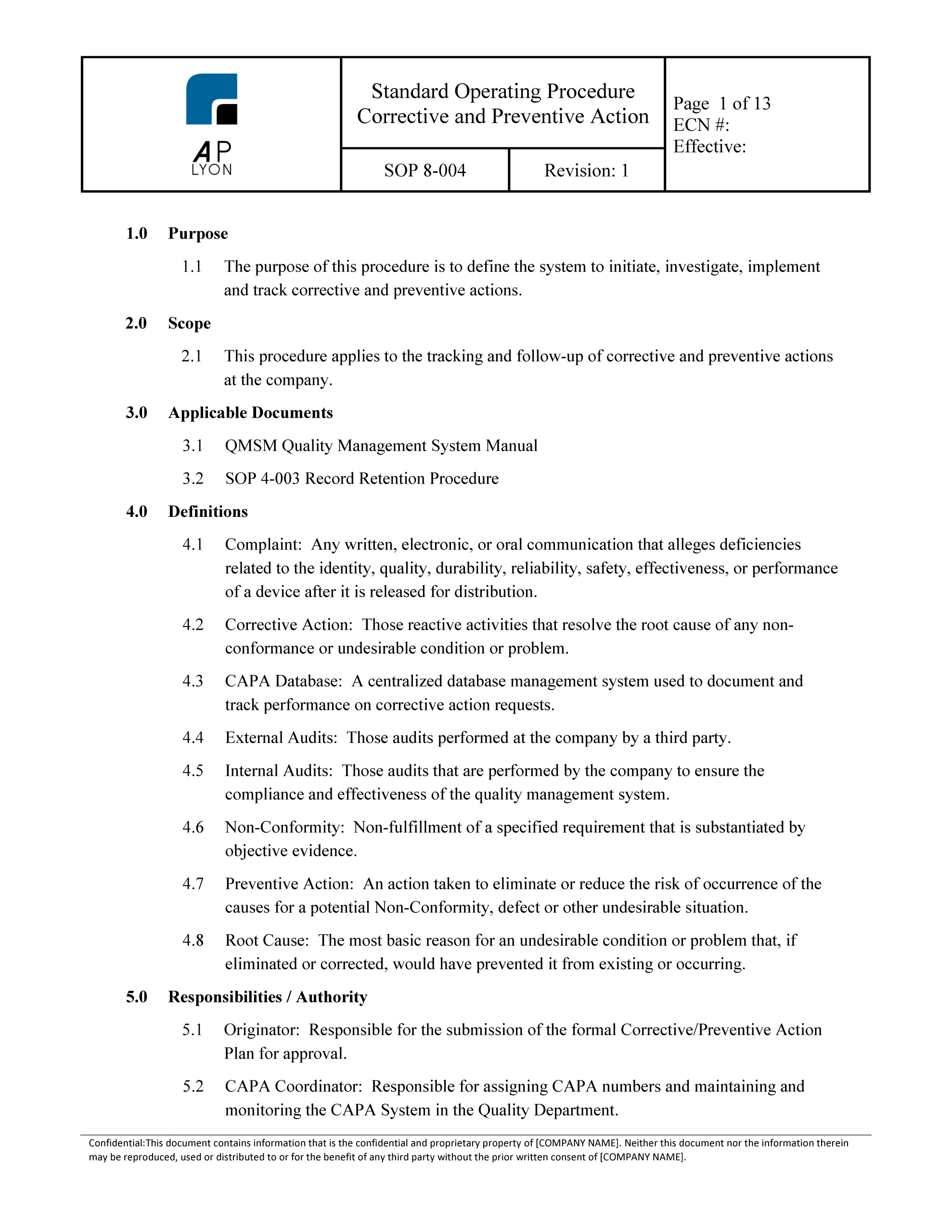
Establishing a systematic approach to managing nonconformities, quality issues, and other potential risks is crucial for maintaining quality and meeting regulatory requirements. A well-defined corrective and preventive action (CAPA) procedure ...
Read more